Crystallography And Defects In Solid ( Manufacturing Engineering – I ) MCQs – Mechanical Engineering MCQs

Latest Mechanical Engineering MCQs
By practicing these MCQs of Crystallography And Defects In Solid ( Manufacturing Engineering – I ) MCQs – Latest Competitive MCQs , an individual for exams performs better than before. This post comprising of objective questions and answers related to “ Crystallography And Defects In Solid ( Manufacturing Engineering – I ) Mcqs “. As wise people believe “Perfect Practice make a Man Perfect”. It is therefore practice these mcqs of Mechanical Engineering to approach the success. Tab this page to check ” Crystallography And Defects In Solid ( Manufacturing Engineering – I )” for the preparation of competitive mcqs, FPSC mcqs, PPSC mcqs, SPSC mcqs, KPPSC mcqs, AJKPSC mcqs, BPSC mcqs, NTS mcqs, PTS mcqs, OTS mcqs, Atomic Energy mcqs, Pak Army mcqs, Pak Navy mcqs, CTS mcqs, ETEA mcqs and others.
Mechanical Engineering MCQs – Crystallography And Defects In Solid ( Manufacturing Engineering – I ) MCQs
The most occurred mcqs of ( ) in past papers. Past papers of Crystallography And Defects In Solid ( Manufacturing Engineering – I ) Mcqs. Past papers of Crystallography And Defects In Solid ( Manufacturing Engineering – I ) Mcqs . Mcqs are the necessary part of any competitive / job related exams. The Mcqs having specific numbers in any written test. It is therefore everyone have to learn / remember the related Crystallography And Defects In Solid ( Manufacturing Engineering – I ) Mcqs. The Important series of Crystallography And Defects In Solid ( Manufacturing Engineering – I ) Mcqs are given below:
Crystallography-1
1. Which of the following has a non-crystalline structure?
a) Iron
b) Quartz
c) Silica glass
d) Tungsten
Answer: c
Explanation: In general, metals exist in a crystalline form. Iron and Tungsten being metals takes up body centered cubic crystalline structure at room temperature. The ceramic compound–silica (SiO2), can exist either in a crystalline form or in a non-crystalline form (amorphous form). While quartz, tridymite and cristobalite are known as its crystalline forms which are being differentiated based on SiO4 tetrahedra linkage style, silica’s non-crystalline (amorphous) form is just called as the silica glass.
2. Which of the following has less crystallinity?
a) Iron
b) Nickel
c) High density polythene
d) Low density polythene
Answer: d
Explanation: It is clear that, iron and nickel being metals possesses a crystalline form, whereas high density polyethylene (HDPE) and low-density ethylene (LDPE) are a class of polymers. These both thermoplastics are semi-crystalline nature, out of which LDPE exhibits a crystallinity of about 50-60% and HDPE of about 90%. Some people may term HDPE as crystalline, but it is more appropriate to restrict it in the category of semi-crystalline class.
3. Which of the following is a characteristic of crystalline structure?
a) High density
b) Low density
c) Range of melting point
d) Short range of order
Answer: a
Explanation: A crystalline structure has very close packing of atoms thus giving rise to high density to material it possesses when compared to its non-crystalline form. For example, quartz being the crystalline form of silica has a density of about 2.65 gm/cm3, whereas its ally–non-crystalline form silica glass has a density of 2.20 gm/cm3. For reference, the other properties being differentiated between crystalline and non-crystalline forms are tabulated below.
| Differentiating Parameter | Structure | |
|---|---|---|
| Crystalline Structure | Non-Crystalline Structure | |
| Geometry | Well defined geometrical shape | Random shape |
| Melting point | Definite melting point | Rage of melting point |
| Density | High density when compared to non-crystalline structure due to strong primary atomic bonding. | Low density when compared to crystalline structure due to weak secondary atomic bonding |
| Range of order | Long range of order (periodicity) | Short range of order (periodicity) |
| XRD Diffraction pattern | Sharp diffraction pattern | Broad hump |
| Examples | Iron, Steel, HDPE, Quartz, etc. | Metallic glass, silica glass, LDPE, etc. |
4. Which of the following is characteristic of non-crystalline structures?
a) Long range of periodicity
b) Well defined structure and geometry
c) Low density
d) Sharp diffraction pattern
Answer: c
Explanation: In non-crystalline structure, there is no definite packing of atoms, which makes them to possess any random shape, further these atoms are being bonded by weak secondary bonds with Van der Wall’s forces, thus giving a low density to material.
5. Which of the following factor is not responsible for the formation of a non-crystalline structure?
a) Atomic packing has open structure
b) Primary bonds are absent
c) Formation of 1-dimensional chain molecule
d) Strong secondary bond
Answer: d
Explanation: A non-crystalline structure is being formed by a secondary bonds or molecular bonds are formed as a result of weak Van der Wall’s of attractions which exist between various atoms. These intermolecular bonds can be further classified as dispersion bonds, dipole bonds, hydrogen bonds, which are all should be considered as weak secondary bonds.
6. Which of the following axis system is being satisfied by cubic crystal system?
a) a = b = c, α = β = γ = 90o
b) a ≠ b = c, α = β = γ = 90o
c) a = b ≠ c, α = β = γ = 90o
d) a = b = c, α ≠ β = ϒ = 90o
Answer: a
Explanation: Simple cubic have all sides equal and all angles equal. For reference, table of 7 Bravais lattices are tabulated below:
| S.No. | Crystal System | Conventional Unit Cell Axis System | |
|---|---|---|---|
| Lengths | Angles | ||
| 1 | Cubic | a = b = c | α = β = γ = 90o |
| 2 | Tetragonal | a = b ≠ c | α = β = γ = 90o |
| 3 | Orthorhombic | a ≠ b ≠ c | α = β = γ = 90o |
| 4 | Rhombohedral (Trigonal) | a = b = c | α = β = γ ≠ 90o |
| 5 | Hexagonal | a = b ≠ c | α = β = 90o, γ = 120o |
| 6 | Monoclinic | a ≠ b ≠ c | α = γ = 90o ≠ β
|
| 7 | Triclinic | a ≠ b ≠ c | α ≠ β ≠ γ ≠ 90o |
*Note—Order trick: C-T-O-R-H-M-T (Decreasing symmetry)
7. Which of the following axis system is being satisfied by tetragonal crystal system?
a) a = b = c, α = β = ϒ = 90o
b) a ≠ b ≠ c, α = β = ϒ = 90o
c) a = b ≠ c, α = β = ϒ = 90o
d) a = b = c, α ≠ β = ϒ = 90o
Answer: c
Explanation: Tetragon has two sides equal and all angles equal.
8. Which of the following axis system is being satisfied by orthorhombic crystal system?
a) a = b = c, α = β = γ = 90o
b) a ≠ b ≠ c, α = β = γ = 90o
c) a = b ≠ c, α = β = γ = 90o
d) a = b = c, α ≠ β = γ = 90o
Answer: b
Explanation: Orthorhombic have all sides unequal and all angles equal to a degree.
9. Which of the following axis system is being satisfied by rhombohedral (trigonal) crystal system?
a) a = b = c, α = β = γ = 90o
b) a ≠ b = c, α = β = γ = 90o
c) a = b ≠ c, α = β = γ = 90o
d) a = b = c, α = β = γ ≠ 90o
Answer: d
Explanation: Rhombohedra have all sides equal and all angles equal but not 90o.
10. Which of the following axis system is being satisfied by hexagonal crystal system?
a) a = b ≠ c, α = β = 90o, ϒ = 120o
b) a ≠ b = c, α = β = ϒ = 90o
c) a = b ≠ c, α = β = ϒ = 90o
d) a = b = c, α ≠ β = ϒ = 90o
Answer: a
Explanation: Hexagonal have two sides equal and two angles equal to 90o and one angle equal to 120o.
11. Which of the following axis system is being satisfied by monoclinic crystal system?
a) a = b = c, α = β = 90o ≠ ϒ
b) a ≠ b = c, α = β = ϒ = 90o
c) a ≠ b ≠ c, α = β = 90o ≠ ϒ
d) a = b = c, α ≠ β = ϒ = 90o
Answer: c
Explanation: Monoclinic have all sides unequal and two angles equal to 90o.
12. Which of the following axis system is being satisfied by triclinic crystal system?
a) a = b = c, α = β = ϒ = 90o
b) a ≠ b = c, α = β = ϒ = 90o
c) a ≠ b ≠ c, α ≠ β ≠ ϒ ≠ 90o
d) a = b = c, α ≠ β = ϒ = 90o
Answer: c
Explanation: Triclinic structures have all sides unequal and all angles are also unequal.
13. Which one of the following is most symmetrical?
a) Simple cubic cell
b) Hexagonal
c) Triclinic
d) Tetragonal
Answer: a
Explanation: In triclinic crystal system, we observe all the sides and angle to equal to each other (a = b = c and α = β = γ = 90o), thus giving highest symmetry (four 3-fold symmetry) among all 7 Bravais Lattices.
14. Which one of the following is least symmetrical?
a) Tetragonal
b) Simple cubic
c) Triclinic
d) Monoclinic
Answer: c
Explanation: In triclinic crystal system, we observe all the sides and angle to unequal to each other (a ≠ b ≠ c and α ≠ β ≠ γ ≠ 90o), thus giving least symmetry (1-fold symmetry) among all 7 Bravais Lattices.
Crystallography-2
1. What is the coordination number of a simple cubic (SC) unit cell?
a) 4
b) 6
c) 8
d) 2
Answer: b
Explanation: There are six nearest neighbouring atoms for every atom in a simple cubic (SC) unit cell, in other words, every atom in a SC unit cell is surrounded by 6 other atoms, thus coordination number of SC unit cell is is 6.
2. What is the coordination number of a face centered cubic (FCC) unit cell?
a) 4
b) 6
c) 8
d) 12
Answer: d
Explanation: In an FCC structure, there are eight atoms, one atom each at the corner of the unit cell and one atom at the centre of each face. For any corner atom of the unit cell, the nearest atoms are face-centred atoms. Thus, the coordination number for an FCC structure = 4 centre atoms below the horizonal plane + 4 centre atoms above the horizontal plane + 4 centre atoms on the horizonal plane.
Hence, the coordination number for an FCC structure is 4 + 4 + 4 = 12.
3. What is the coordination number of body centered cubic unit cell?
a) 4
b) 6
c) 8
d) 2
Answer: c
Explanation: For any corner atom of the BCC unit cell, the nearest atoms are the body centred atoms. There are eight-unit cells in neighbours with body-centered atoms. Hence, the coordination number for a BCC cubic unit cell is 8.
4. Effective number of atoms in a simple cubic (SC) unit cell is equal to _________
a) 4
b) 1
c) 8
d) 2
Answer: b
Explanation: Total number of atoms at corners = 8 and each corner atom is shared by total 8-unit cells. Thus, effective number of atoms in an SC unit cell: 8 × 8⁄8 = 1.
5. Effective number of atoms in a face centered cubic (FCC) unit cell is equal to ________
a) 4
b) 1
c) 8
d) 2
Answer: a
Explanation: In an FCC unit cell, there are eight atoms: one at each corner of the cube and six face centered atoms of the six planes of the cube. As corner atoms are shared by eight adjacent cubes and the face centered atoms by two adjacent unit cells, total effective number of atoms in an FCC unit cell will be 4.
6. Effective number of atoms in a body centered cubic (BCC) unit cell is equal to _____________
a) 4
b) 6
c) 1
d) 2
Answer: d
Explanation: The unit cell of a cube contains eight atoms at the corners, which are shared by the eight adjoining cubes and one atom at the centre of the cube.
8 atoms at the corner: (8×1⁄8) = 1 atom + 1 centre atom in the unit cell, So, there are “2” effective number of atoms in a BCC unit cell.
7. The atomic packing fraction in a simple cubic unit cell is ________
a) 0.74
b) 0.52
c) 0.68
d) 0.66
Answer: b
Explanation: a=r and APF = (volume of effective number of atoms/volume of unit cell).
8. The atomic packing fraction in a body centered cubic unit is cell is ________
a) 0.74
b) 0.52
c) 0.68
d) 0.66
Answer: c
Explanation: r=(√3)/4×a and APF = (volume of effective number of atoms/volume of unit cell).
9. If the radius of a copper atom is given as 1.27 Ao, its density (in kg/m3) will be?
a) 100.01
b) 86.25
c) 8979
d) 7968
Answer: c
Explanation: Formula to calculate the density of a cubic metal:
ρ (kg/m3) = \(\frac{n × A.W}{a^3}\) × 1.66 × 10-27
[where, ρ = density of metal, n = effective number of atoms per unit cell, A.W = Atomic weight of the metal in amu and a = lattice parameter in meter] Given: radius of copper = 1.27 Ao = 1.27×10-10 m
We know that atomic weight of copper = 63.5 amu
Lattice parameter to atomic radius relation for cubic structures are as follows:
| Crystal Structure | Effective Number of Atoms per Unit Cell | Effective Number of Atoms per Unit Cell |
|---|---|---|
| Simple Cubic (SC) | a = 2r | 1 |
| Body Centered Cubic (BCC) | a = 4r/√3 | 2 |
| Face Centered Cubic (FCC) | a = 4r/√2 | 4 |
| Hexagonal Close Packed Cubic (HCP) | a = 2r | 6 |
We know that copper has FCC crystal structure, so it has ‘4’ effective number of atoms per unit cell and given its atomic radius = 1.27×10-10 m
Therefore, lattice parameter of copper (a) = (4×1.27×10-10)/√2 = 3.59×10-10 m
Therefore, density of copper = \(\frac{4 × 63.5}{(3.59 × 10^{-10})^3}\) × 1.66 × 10-27 ≈ 8979 kg/m3.
10. The atomic packing fraction in a face centered cubic unit is?
a) 0.74
b) 0.52
c) 0.68
d) 0.66
Answer: a
Explanation: a=2√2×r and APF = (volume of effective number of atoms/volume of unit cell).
Miller Indices
1. Stacking sequence in hexagonal close packed (HCP) structure is?
a) AAAAA
b) ABABAB
c) ABCABC
d) AABBAA
Answer: b
Explanation: The geometry of close packed hexagonal unit cell can be understood from the below figure, which indicates a plane view of atoms.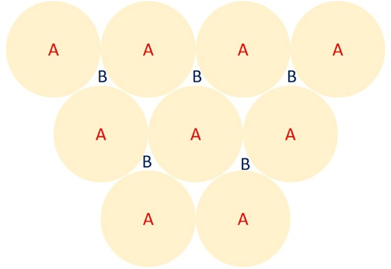
Figure: Stacking sequences of close packed layers of atoms. A-first layer (with outlines of atoms shown in gold colour); B-second layer.
A is the first layer (with the circular outlines of the atoms drawn in gold colour) and B is the second layer (outlines of the atoms not shown for clarity). In hexagonal close packed unit cell, the third layer of atoms go directly above the A layer, the fourth layer over the B layer, and so on; the sequence becomes ABABAB.
2. Stacking sequence in face centered cubic (FCC) close packed structure is?
a) AAAAA
b) ABABAB
c) ABCABC
d) AABBAA
Answer: c
Explanation: The stacking sequence in FCC can be best understood from the below figure.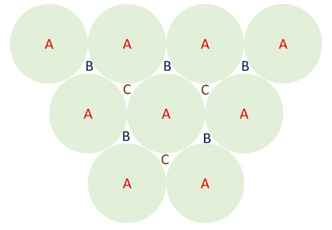
Figure: Stacking sequences of close packed layers of atoms. A-first layer (with outlines of atoms shown in green colour); B-second layer; C-third layer.
A is the first layer (with the circular outlines of the atoms drawn in green colour) and B is the second layer (outlines of the atoms not shown for clarity). The third layer of atoms goes above the interstices marked C and the sequence only repeats at the fourth layer, which goes directly above the first layer. Thus, the stacking sequence is now ABCABC.
3. For pane (1 1 1) of FCC having a lattice parameter ‘a’, planar atomic density is given by?
a) 2.31/a2
b) 2.31/a3
c) 1.31/a2
d) 1.31/a3
Answer: a
Explanation: Upon visualizing (1 1 1) plane of FCC, one can identify that there the equilibrium triangle (1 1 1) of FCC consists 2 atoms (1⁄6×3 + 1⁄2×3=2). Also, the area of the equilibrium triangle (1 1 1) is 0.886 a2.
Therefore, the number of atoms per square inch which is nothing but its planar density = \(\frac{2}{0.866 a^2} = \frac{2.31}{a^2}\).
4. For pane (1 1 1) of BCC having a lattice parameter ‘a’, planar atomic density is given by?
a) 1.07/a2
b) 0.58/a2
c) 2.07/a2
d) 0.78/a2
Answer: b
Explanation: From the geometry of the triangle (1 1 1), it is clear that it has 0.5 atoms in it (1/6×3 = 0.5) and the area of the triangle (1 1 1) is 0.866 a2.
Therefore, the number of atoms per square inch which is nothing but its planar density = \(\frac{0.5}{0.866 a^2} = \frac{0.58}{a^2}\).
5. For pane (1 0 0) of BCC having a lattice parameter ‘a’, planar atomic density is given by?
a) 1/a3
b) 2/a2
c) 3/a4
d) 1/a2
Answer: d
Explanation: The atomic arrangement on square (1 0 0) of BCC indicates that it has 1 atom and area of this square (1 0 0) is a2. Thus, its planar density = 1/a2.
6. For pane (1 1 0) of BCC having a lattice parameter ‘a’, planar atomic density is given by?
a) 3.690/a2
b) 2.312/a2
c) 1.414/a2
d) 0.580/a2
Answer: c
Explanation: From the geometry of the rectangle (1 1 0), it is clear that it has 2 atoms in it (1⁄4×4+1=2) and the area of the rectangle (1 1 0) is 0.866 a2.
Therefore, the number of atoms per square inch which is nothing but its planar density = \(\frac{2}{1.414 a^2} = \frac{1.414}{a^2}\).
7. For pane (1 1 0) of SC having a lattice parameter ‘a’, planar atomic density is given by?
a) 0.508/a2
b) 0.707/a2
c) 0.707/a3
d) 0.508/a3
Answer: b
Explanation: The atomic arrangement on (1 1 0) plane in simple cubic (SC) unit cell indicates that it has 1 atom (1⁄4×4=1) in this plane having an area = \(\sqrt{2} a^2\).
Therefore, the number of atoms per square inch which is nothing but its planar density = \(\frac{1}{1.414 a^2} = \frac{0.707}{a^2}\).
8. For pane (1 1 1) of SC having a lattice parameter ‘a’, planar atomic density is given by?
a) 0.58/a2
b) 0.78/a3
c) 0.68/a2
d) 0.88/a2
Answer: a
Explanation: The atomic arrangement on triangle (1 1 1) in simple cubic (SC) unit cell indicates that it has 0.5 atoms (1⁄6×3=0.5) in this plane having an area = 0.866 a2.
Therefore, the number of atoms per square inch which is nothing but its planar density = \(\frac{0.5}{0.866 a^2} = \frac{0.577}{a^2}\).
9. Which of the following equation describes Bragg’s law of diffraction? (Assume that all symbols have their usual meaning.)
a) 2d sinθ = λ
b) 2d = nλ
c) 2d = nλ sinθ
d) 2d sinθ = nλ
Answer: d
Explanation: Bragg’s law, which describes the constructive interference conditions for θ to be at its strongest: nλ = 2d sinθ, where λ is the wavelength of the incident wave and and n is the order of diffraction which is always a positive integer and d is the interplanar spacing and θ = angle of diffraction. This equation can be easily derived upon constructing a basic wave reflection diagram taking incident angle as θ.
10. Powder X-ray technique can be used to determine crystal structures.
a) True
b) False
Answer: a
Explanation: The powder method is used to determine the values of lattice parameters accurately. As we know, lattice parameters defines the axis system, which in turns can predict the crystal structure of the powder sampled.
Imperfection and Defects In Solid
1. Vacancy defects in solids is a sub type of __________
a) Point imperfections
b) Line imperfections
c) Volume imperfections
d) Surface imperfections
Answer: a
Explanation: The simplest of the point defects is a vacancy, vacant lattice site. This could occur an atom is being missing from its lattice site. All crystalline solids contains vacancies, in fact, it is impossible for a material to be free of these vacancies, making them to call equilibrium defects. For reference, all the imperfections in solids are being tabulated below:
| IMPERFECTIONS IN SOLIDS | |||
|---|---|---|---|
| Point Imperfections (Zero-Dimensional) | Line Imperfections (One-Dimensional) | Surface Imperfections (Two-Dimensional) | Volume Imperfections(Three-Dimensional) |
| Vacancies | Dislocations | Surface | Voids |
| Interstitial Impurities | Disclinations | Grain boundaries | Cracks |
| Substitutional Impurities | Twin boundaries | Inclusions | |
| Schottky defect | Stacking faults | Precipitates | |
| Frenkel defect | Interphase boundary | Twins | |
2. Substitution of a foreign atom in the site of parent atom in the crystal is a?
a) Vacancy defect
b) Substitution impurity
c) Volume imperfection
d) Vacancy defect
Answer: b
Explanation: When an impurity atom of equal to atomic size of the host atom is being replaced or substituted for the host atoms, is called substitutional impurity.
3. Edge dislocation imperfection is a sub type of _____________
a) Point imperfections
b) Line imperfections
c) Volume imperfections
d) Surface imperfections
Answer: b
Explanation: A dislocation is a one-dimensional or linear defect around which some of the atoms are misaligned. Edge dislocation is a type of dislocation, where an extra portion of plane of atoms, the edge terminates within the crystal.
4. Displacement of an ion from regular location to interstitial location is known as ____________
a) Vacancy defect
b) Line imperfection
c) Schottky’s defect
d) Frenkel defect
Answer: d
Explanation: Frenkel defect, named after its discoverer “Yakov Frenkel”, forms when an atom leaves its regular position thus creating a vacancy there, and becomes interstitial by positioning itself into nearby interstitial location. Usually small ion (cation) undergoes this phenomenon.
5. When a pair of cation and anion are missing in a crystal, it is called ____________
a) Vacancy defect
b) Line imperfection
c) Schottky’s defect
d) Frenkel defect
Answer: c
Explanation: Schottky’s defect, named after its discoverer “Walter H. Schottky”, forms when two atoms of opposite charge (anion and cation) leaves their regular atomic positions thus creating two vacancies. As two atoms of opposite charges are leaving, there is no change in overall charge of the material with Schottky’s defect. Schottky’s defect is known for its presence in ionic crystals.
6. Which one of the following is not a zero-dimensional defect?
a) Vacancy defect
b) Substitution imperfection
c) Schottky’s defect
d) Screw dislocation
Answer: d
Explanation: Screw dislocation is a type of dislocations (linear or one-dimensional defects) which thought to be formed by a shear stress that is applied to produce the distortion in a spiral manner inside the crystal, indicating burgers vector parallel to this dislocation type.
7. Twin or Twinning is a category of ________
a) Point imperfections
b) Line imperfections
c) Volume imperfections
d) Surface imperfections
Answer: c
Explanation: A twin boundary is a two-dimensional imperfection (surface imperfection), but the entire twin is a category of volume imperfections.
8. As the grain size of a metal increases, its strength ________
a) Decreases
b) Increases
c) Remains constant
d) No effect of grain size on strength
Answer: a
Explanation: Strength of a metal is directly proportional to its ability resist plastic deformation, thus resisting its dislocation movement. As one can relate that, more the number of grain boundaries (fine is the grain size), more is the obstruction to the movement of dislocation, thus more is the strength of a metal. So, if grain size increases, its strength decreases. This phenomenon can be best understood with the help of Hall-Petch relation.
9. As the grain size of a meal increases, its ductility ________
a) Decreases
b) Increases
c) Remains constant
d) No effect of grain size on ductility
Answer: a
Explanation: Fine the grain size (lower the grain size), more is the number of grain boundaries, thus more is the yield strength, thus more is the ductility. So, if grain size increases, its ductility decreases.
10. Phenomenon of cross slip occurs in ________
a) Point imperfections
b) Line imperfections
c) Volume imperfections
d) Surface imperfections
Answer: b
Explanation: When we apply a stress, it causes screw dislocations to move from one slip plane to another slip plane, which is known as its cross slip. Cross slip is one of the primary mechanisms that causes plastic deformation in metals.
