States Of Matter ( Chemistry ) MCQs – Latest Chemistry MCQs
Latest Chemistry MCQs
By practicing these MCQs of States Of Matter ( Chemistry ) MCQs – Latest Competitive MCQs , an individual for exams performs better than before. This post comprising of objective questions and answers related to “ States Of Matter ( Chemistry ) Mcqs “. As wise people believe “Perfect Practice make a Man Perfect”. It is therefore practice these mcqs of Chemistry to approach the success. Tab this page to check ” States Of Matter ( Chemistry )” for the preparation of competitive mcqs, FPSC mcqs, PPSC mcqs, SPSC mcqs, KPPSC mcqs, AJKPSC mcqs, BPSC mcqs, NTS mcqs, PTS mcqs, OTS mcqs, Atomic Energy mcqs, Pak Army mcqs, Pak Navy mcqs, CTS mcqs, ETEA mcqs and others.
Chemistry MCQs – States Of Matter ( Chemistry ) MCQs
The most occurred mcqs of States Of Matter ( ) in past papers. Past papers of States Of Matter ( Chemistry ) Mcqs. Past papers of States Of Matter ( Chemistry ) Mcqs . Mcqs are the necessary part of any competitive / job related exams. The Mcqs having specific numbers in any written test. It is therefore everyone have to learn / remember the related States Of Matter ( Chemistry ) Mcqs. The Important series of States Of Matter ( Chemistry ) Mcqs are given below:
Intermolecular Forces
1. What are the forces of attraction and repulsion between interacting molecules known as _________
a) attractive forces
b) repulsive forces
c) intermolecular forces
d) intramolecular forces
Answer: c
Explanation: Intermolecular means between molecules and intramolecular means within in molecule. Both attractive and repulsive forces include intermolecular forces. Therefore, the forces of attraction and repulsion between interacting molecules are known as Intermolecular forces.
2. Deviation of real gas behavior from ideal gas is discovered by _____________
a) Jonathan
b) Van der Waals
c) Boyle
d) Newland
Answer: b
Explanation: A Dutch scientist named Johannes van der Waals found out the reason for real gas behavior’s deviation from ideal gas behavior. And he said that those forces which were named van der Waals were responsible.
3. London force is also known as _____________
a) dispersion force
b) van der Waals forces
c) hydrogen bonding
d) covalent bonds
Answer: a
Explanation: Electrically symmetrical atoms and non-polar molecules having zero dipole movement due to their electron distribution may sometimes develop a momentary dipole also known as London force or the Dispersion forces.
4. Dispersion force’s interaction energy is proportional to (take “r” as the distance between the two particles) ___________
a) r6
b) 1/r-6
c) r2
d) 1/r6
Answer: d
Explanation: The interaction energy between two atoms or molecules experiencing dispersion or London forces is inversely proportional to the sixth power of the distance between those molecules when found, experimentally.
5. Dipole-Dipole forces are stronger than _______ and weaker than _________ interactions.
a) dipole-induced dipole, london
b) ion-ion, dispersion
c) ion-ion, london
d) london, ion-ion
Answer: d
Explanation: Dipole-Dipole forces are stronger than London forces and weaker than ion-interactions. As only the partial charges are involved. London forces have no charges, and ion-ion forces have full charges.
6. HCl is an example of __________
a) dipole-dipole intercations
b) dipole-induced dipole interactions
c) london intercation
d) van der waals interaction
Answer: a
Explanation: Dipole-Dipole interactions occur between molecules having permanent dipoles. And also the ends of dipoles posses partial charge which represented by a Greek letter δ. In an HCl molecule, the same type of interactions occur.
7. Take “r” as the distance between two molecules. The energy between stationary polar molecules is proportional to ____________ in the case of dipole-dipole intercations.
a) 1/r3
b) r3
c) r2
d) 1/r2
Answer: a
Explanation: The energy between two polar molecules which are stationary, is inversely proportional to the cube of the distance between the molecules, in the case of dipole-dipole intercations. This interaction is stronger than the London forces.
8. Which of the following interaction occurs between a permanent dipole and a neutral molecule?
a) Dipole-Dipole interactions
b) Dipole-induced dipole interactions
c) London interaction
d) Van der Waals interaction
Answer: b
Explanation: Dipole-induced dipole interactions occur between a molecule of permanent dipole and molecule lacking permanent dipole. The dipole gets induced to the other molecule in this particular interaction.
9. Hydrogen bond plays a vital role in determining substance properties and structure. Which of the following may not an example?
a) Proteins
b) Nucleic acids
c) Methane molecule
d) Water
Answer: c
Explanation: As per the above statement, substance properties and structure is determined as per the hydrogen bond present in them, but in a methane molecule, there is no hydrogen bond. So it may not be an example.
10. Molecules do exert repulsive forces on one other.
a) True
b) False
Answer: a
Explanation: When two molecules come closer, the electron clouds between them they repel. This is the reason why solids and liquids cannot be easily compressed. As the distance between molecules decreases, the repulsive forces become much stronger.
Thermal Energy
1. Which energy is generated from due to the motion of its particles?
a) Thermal
b) Muscular
c) Momentary
d) Potential
Answer: a
Explanation: Thermal energy is the result of the motion of a body’s atoms and molecules. It depends on the temperature and is directly proportional to each other. It’s the average of kinetic energy and is responsible for a body’s motion.
2. Which of the following posses highest thermal energy?
a) Water at 0°
b) Iron at 37°
c) Milk at 50°
d) Chocolate at 26°
Answer: c
Explanation: As we know that, the thermal energy is dependent on temperature, among the options milk at given temperature has a higher temperature, so milk posses higher thermal energy than others (temperature is proportional to thermal energy).
3. Thermal energy is an example of __________
a) kinetic energy
b) potential energy
c) muscular energy
d) momentary energy
Answer: a
Explanation: Thermal energy is also defined as an average measure of kinetic energy. It is a result of a body’s motion and that of its atoms and molecules. Thermal energy, which is an example of kinetic energy is responsible for a body’s motion.
4. The faster the body moves, the higher the thermal energy.
a) True
b) False
Answer: a
Explanation: As thermal energy is a type of kinetic energy, the faster the object moves, the higher the thermal energy. The measure of an average of kinetic energy is thermal energy. So the above statement regarding thermal energy is true.
5. ___________ of particles results in the rise of thermal energy.
a) Direction
b) Vibration
c) Shape
d) Atoms
Answer: b
Explanation: Temperature increases due to the vibration of particles. As we know that temperature is directly proportional to the thermal energy, the vibration of particles results in the rise the thermal energy.
6. Which of the following may not be a source of thermal energy?
a) Micro-oven
b) Sun
c) Moon
d) Heater
Answer: c
Explanation: Thermal energy is also known as heat energy, it’s source s are the same as heat energy. As we know that micro-oven produces heat waves to raise food’s temperature and bake them, sun for solar energy and heater produces heat. Moon doesn’t.
7. Choose the best option. Boiling kettle is an example of ____________
a) only thermal energy
b) both thermal and Kinetic energy
c) only kinetic energy
d) potential and Kinetic energy
Answer: b
Explanation: A boiling kettle produces heat, and causes the liquid’s temperature to rise. As the temperature rises, it results in thermal energy, but as kinetic energy is a type of kinetic energy, the answer both thermal and kinetic energy is the best option.
8. Thermal energy transfer can occur through ______ ways.
a) 2
b) 1
c) 3
d) 0
Answer: c
Explanation: Thermal can transfer through ways, there are namely conduction, convection, and radiation. Conduction needs direct contact of particles, convection is possible to fluids and radiation through electromagnetic waves.
Chemical Bonding And Moleculer Structure MCQs
9. In which of the following particles, convection is not possible?
a) Milk
b) Water
c) Atmosphere
d) Iron
Answer: d
Explanation: Convection is a way of transferring thermal energy. It is possible in fluids only. Fluids include liquids and gases. Milk and water are liquids, whereas the atmosphere is a gas. So convection is not possible in iron, which is a solid.
10. Sunlight and heat reaching earth is an example of _________
a) conduction
b) radiation
c) convection
d) both convection and conduction
Answer: b
Explanation: Thermal energy is transferred in the form of heat and sunlight. This process occurs through radiation, which is a type of transfer of thermal energy. It requires no direct contact and uses electromagnetic waves.
Intermolecular Forces vs Thermal Interactions
1. What is the result of balancing between intermolecular forces and thermal energy?
a) matter
b) three States of matter
c) four States of matter
d) chemical bond formation
Answer: b
Explanation: In a molecule, intermolecular forces always tend to keep molecules with each other while the thermal energy always tries to separate them. So the result of balancing between intermolecular energy and thermal energy is three States of matter.
2. Which state of matter is likely to form when there is a predominance of intermolecular energy?
a) solid
b) liquid
c) gas
d) both solid and gas
Answer: a
Explanation: When there is a predominance of intermolecular energy in the matter, the order of states of matter that are the likely to form is solids, liquids and then gases. It follows reverse order in case of the predominance of thermal energy.
3. A gas can be liquefied only through an increase in compression.
a) true
b) false
Answer: b
Explanation: The above statement is false because gas cannot be liquefied only through compression, even the temperature needs to be lowered in order to compress the gas. This is because of the reaction of intermolecular forces and thermal energy between the molecules.
4. Gas is readily formed in case of predominance of ___________
a) intermolecular energy
b) thermal energy
c) both intermolecular energy and thermal en energy
d) neither intermolecular energy nor thermal energy
Answer: b
Explanation: In the case of the predominance of thermal energy, the gas is likely to be formed rather than liquids and solids. This happens because the thermal energy tends to separate the molecules in a substance.
5. In the case of ice which energy do you think that is predominant?
a) Thermal energy
b) Intermolecular energy
c) Intermolecular energy
d) Heat energy
Answer: b
Explanation: In case of ice the molecules are tightly packed and we do know that in solid the intermolecular forces are so high that they are tightly packed so intermolecular energy is predominant in case of ice.
6. The following are the temperatures of milk in Celsius. In which of the following, do you think intermolecular forces are predominant than thermal energy?
a) 35
b) 82
c) 50
d) 9
Answer: d
Explanation: The rise in temperature depicts thermal energy. So among the given options at 82 degrees Celsius the milk has the highest thermal energy and at 9 degrees Celsius the milk has higher intermolecular energy. So at 9 degrees, the intermolecular forces are predominant than thermal energy in milk.
7. What is a term used for the conversion of solid into gas directly?
a) Evaporation
b) Sublimation
c) Condensation
d) Melting
Answer: b
Explanation: The process of direct conversion of solid to gases is sublimation while the process of conversion of liquid to gas is evaporation whereas the process of conversion of solid to liquid is melting and liquid to solid is condensation.
8. Particles in a solid __________
a) are tightly packed
b) are loosely packed
c) move continuously
d) collide with each other
Answer: a
Explanation: When there is a predominance of intermolecular energy instead of thermal energy, the molecules are tightly packed and have a definite shape and structure; these are called solids. So in solid, the particles are tightly packed.
9. Compressibility is high in case of ___________
a) solids
b) liquids
c) gases
d) both solids and liquids have the same amount of compressibility
Answer: c
Explanation: Compressibility is high in the case of gases because thermal energy is predominant in gases. This energy enables the molecules of gases to move away from each other so that when compressed together they compress easily by reducing the distance between each other comparatively.
10. During the process of freezing the temperature is _________
a) constant
b) increasing
c) decreasing
d) irregular
Answer: a
Explanation: During the process of freezing the water, water converts into ice that means new bonds are creating during this process and the temperature remains constant but the internal energy changes during this phase.
Gaseous State
1. What is the lowermost layer of the earth?
a) stratosphere
b) troposphere
c) ionosphere
d) mesosphere
Answer: b
Explanation: It is the lowest layer of the earth’s atmosphere and is held to the earth by the gravitational force. It is very vital for human life and it protects us from harmful radiation. It contains important molecules like dioxygen, carbon dioxide, water vapor, etc.
2. Which of the following statement is true regarding gases?
a) gases are highly incompressible
b) gases exert equal pressure on each and every direction
c) its volume and shape is fixed
d) gases have the highest density among the 3 States of matter
Answer: b
Explanation: The correct statement is that gases exert equal pressure on each and every direction. Among the given options the corrected statements of other options are that gases are highly compressible, they occupy the shape and volume of the container as they have no fixed shape and volume and also that they have the least density among the three states of matter.
3. Which of the following element is not a gas?
a) Hydrogen
b) Oxygen
c) Mercury
d) Nitrogen
Answer: c
Explanation: Mercury is not a gas, it is a liquid in room temperature and it is a metal. There are 11 gases which are gases at room temperature they are hydrogen, Nitrogen, Oxygen, fluorine, chlorine, Helium, Neon, Argon, Krypton, Xenon, and Radon.
4. Gases have low density than that of solids and liquids because of __________
a) no thermal energy
b) higher intermolecular energy
c) both intermolecular energy and thermal energy are the same
d) higher thermal energy
Answer: d
Explanation: In gases, there is less amount of intermolecular energy and higher amount of thermal energy. As we know that thermal energy separates some molecules from one another so gases have low density than that of solids and liquids.
5. Gases mix properly without any mechanical aid.
a) true
b) false
Answer: a
Explanation: As the forces of interaction between molecules of a gas is negligible when compared with solids and gases. They mix properly because of higher thermal energy and lower intermolecular energy, so the above statement is true.
6. Which of the following is not a gas law?
a) Boyle’s law
b) Charles law
c) Hooks law
d) Gay lussac’s law
Answer: c
Explanation: Boyle’s law is about the relationship between pressure and volume while Charles law about temperature and volume. Gay lussac’s law is about pressure-temperature relationship & hooks law is a law that is in Physics relating to stress.
7. What is the percentage of Nitrogen in the atmosphere approximately?
a) 78.09
b) 21
c) 20
d) 32
Answer: a
Explanation: That composition of earth’s atmospheric gases is as follows; 78.09 percent of Nitrogen, 24.95 percent of oxygen, 0.93 percent of argon, 0.04 percentage of carbon dioxide and a small amount of water vapor and other gases in the atmosphere.
8. What can you say about particles motion in gases?
a) only vibratory
b) very slow
c) both vibratory and irregular
d) too Rapid and random
Answer: d
Explanation: A particle’s motion in the gaseous state is too rapid and random while in solids it’s restricted to vibratory motion and in liquids, it’s very slow. This is one of the very basic properties of substances in the gaseous state.
Classification Of Elements And Periodocity In Properties MCQs
9. How many moles of oxygen are present in 64 grams of oxygen?
a) three moles
b) two moles
c) one mole
d) 16 moles
Answer: b
Explanation: As we know that the number of moles of a gas is given by the amount of the substance in weight divided by the molecular weight of the substance. So in case of oxygen, it is 64 grams divided by 32 grams and that is two moles.
10. At STP conditions how much volume does one mole of a gas comprise of _________
a) 22.4 liters
b) 24 liters
c) depends on the molecular weight of the gas
d) depends on some other conditions
Answer: a
Explanation: Every one Mole of gas at STP consists of 22.4 liters of volume, that is at 0° Celsius of temperature and one-atmosphere pressure or 76 mm of Mercury pressure. Also, note that one mole of a gas is the amount of gas in weight divided by the molecular weight of the gas.
Gas Laws
1. At a constant temperature, the pressure of a gas is given as one atmospheric pressure and 5 liters. When the atmospheric pressure is increased to 2 atm, then what is the volume of the gas?
a) 1 liter
b) 5 liters
c) 10 liters
d) 2.5 liters
Answer: d
Explanation: As we know that, Boyle’s law states at a constant temperature, the pressure of a gas is inversely proportional to its volume so P1V1 equals to P2V2 by substituting P1 as one atmospheric pressure V1 as 5 liters P1 as to atmospheric pressure we get V2 as 5/2 that is 2.5 liters.
2. What is the shape of the graph that is drawn between pressure and volume?
a) A straight line
b) Circular
c) Parabola
d) Hyperbola
Answer: d
Explanation: Boyle’s law states that at constant temperature pressure is inversely proportional to the volume of gas so here the graph between the pressure as y-axis volume as x-axis is in the shape of a hyperbola.
3. What is the name of the graph that is drawn, when the temperature is kept constant?
a) Isotherm
b) Isochoric and isobar
c) Isochoric
d) Isobar
Answer: a
Explanation: The graphs with constant temperature plot are isotherms. For example, the graph that is used to detect the Boyle’s law, that is between pressure and volume is an isotherm as the temperature is constant in this graph.
4. There is a ball that will burst if the pressure exceeds 0.12 bars. The pressure of the gas is 1 bar and the volume is 2.5 liters. What can be the maximum volume that the ball can be expanded?
a) 0.12 liters
b) 2.5 liters
c) 0.3 liters
d) 1 liter
Answer: c
Explanation: According to Boyle’s law at a constant temperature, the pressure is inversely proportional to the temperature so here P1V1 is equaled to P1V2 by equating P1V1 is equaled to 1 x 2.5 = 2.5, so the maximum volume of the ball that can be expanded is 2.5/0.12 =0.3 liters.
5. How much does the volume of the gas increase if we increase the temperature by 1 Degree?
a) 273 liters
b) 1 by 273rd of the original volume of the gas
c) 1 liter
d) Hundred liters
Answer: b
Explanation: According to Charles law, the volume of the fixed gas at constant pressure is directly proportional to the Absolute Temperature of the gas. So we thereby represent this as Vt = V0(1 + t/273) where Vt is the volume of the gas at temperature t and V0 is the volume of the gas at 0 degrees Celsius that is absolute temperature.
6. There is a balloon filled with a gas at 26-degree centigrade and has a volume of about 2 liters when the balloon is taken to a place which is at 39-degree centigrade, what would be the volume of the gas that is inside the balloon?
a) 2 liters
b) 3 liters
c) 1.5 liters
d) 0.67 liters
Answer: b
Explanation: As we know that temperature is directly proportional to the volume at constant pressure, 26/39 = 2/ X; so here by equating X equals to 3 liters. Hence required a volume of the balloon at 39 degrees is 3 liters.
7. By observing the below-given figure which of the options do you think is the correct one?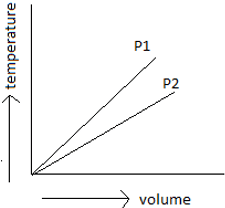
a) P1 is greater than P2
b) P2 is greater than P1
c) P1 is equal to P2
d) P1 may be equal to P2
Answer: a
Explanation: By using Boyle’s law, draw a parallel line to volume axis, so as to maintain a constant temperature. Draw perpendicular lines to the point of intersection of pressure lines to constant temperature and volume axis. Now see to the lower the volume, higher the pressure. So P1 is greater than P2.
8. An ideal gas of 10 moles occupies _________ volume.
a) 22.4 liters
b) 2.24 liters
c) 224 liters
d) 2240
Answer: c
Explanation: As we know that the number of moles is proportional to the volume as per Avogadro’s law and we also know that an ideal gas at STP occupies 22.4 liters of volume. So here 10 moles of gas occupies 224 liters of volume.
9. When a graph is drawn between the pressure and temperature of the gas it is known as _________
a) isochoric
b) isobar
c) isotherm
d) isotopic
Answer: a
Explanation: When a graph is plotted between pressure on y-axis and temperature on x-axis straight line is formed at a constant volume and this graph is known as isochoric. As we know that gay lussac’s law proposes that at the constant volume the pressure and temperature are directly proportional.
10. At 22 degree Celsius a gas consists of pressure 1.1 bars then what is the temperature when the gas consists a pressure of 2.2 bars?
a) 11 degree Celsius
b) 44 degree Celsius
c) 33 degree Celsius
d) 22 degree Celsius
Answer: b
Explanation: According to Gay-Lussac’s law, at the constant volume, the pressure is directly proportional to the temperature of gas so P1/P2 = T1/T2 that is 1.1 Bar/2.2 bar = 22 degrees Celsius/44 degree Celsius. So the temperature required is 44 degrees Celsius.
Ideal Gas Equation
1. What is the constant in ideal gas equation known as?
a) Universal gas constant
b) Pressure constant
c) Temperature constant
d) Boltzmann constant
Answer: a
Explanation: The ideal gas equation is given by PV = nRT where P is pressure, V is volume, n is the number of moles, T is the temperature in Kelvin and R is given by universal gas constant its value is 8.314kgm2s-2.
2. A certain gas occupies 200 ml of volume at 2 bar pressure at hundred degrees Kelvin. How much volume does it occupy at 5 bar pressure and 200 degrees Kelvin?
a) 200 ml
b) 160 ml
c) 240 ml
d) 320 ml
Answer: b
Explanation: From ideal gas law, we know that P1V1/T1 = P2V2/T2. Here we take P1 as 2 bar, V1 as 200 ml, T1 as hundred degrees Kelvin, P2 as 5 bar and T2 as 200 degrees Kelvin, so by substituting the above values 200 x 2/100 = 5 x V2/200; V2 = 160 ml.
3. Which of the following do you think is a correct relationship between the molar mass of gas temperature and its pressure?
a) M = dRT/P
b) M = dRT/V
c) PV = nRT
d) M = VRT/P
Answer: a
Explanation: We know that the ideal gas equation is given by PV = nRT. Number of moles = n; can also be written as m/M, so PV = nRT becomes, PV = mRT/M. We also have density d = m/V, so molar mass M = dRT/P.
4. If the pressure of dry gas is given by X and the total pressure is given by X + 3, then what is aqueous tension?
a) 2
b) X
c) X + 2
d) 3
Answer: d
Explanation: As we know that the pressure of the dry gas is given by the difference between the total pressure and the Aqueous tension, so aqueous tension equals x + 3 – x = 3. So the aqueous tension is three units.
5. In a cylinder of pressure 1 bar, there is the hydrogen of 20 grams and neon of 50 grams, what is a partial pressure of hydrogen?
a) 0.2
b) 0.8
c) 0.4
d) 0.6
Answer: b
Explanation: As we know that the partial pressure of a gas is the product of its mole fraction and the total pressure of the gases present, so here the partial pressure of hydrogen is 10/10 + 2.5 = 0.8. Mole fraction is given by the number of moles of required as per the total number of the gas moles.
6. If the partial pressure of oxygen is given by three bar and the partial pressure of the other gas is four bar, then what is a total pressure that is exerted?
a) 7 bar
b) 3 bar
c) 4 bar
d) 1 bar
Answer: a
Explanation: The total pressure that is exerted is given by the sum of the partial pressure of the gases present. By adding, the sum of the partial pressures is 3bar + 4bar = 7bar, so the total pressure is 7 bar in this case.
7. Who gave the law regarding the partial pressure?
a) Charles
b) Dalton
c) Lussac
d) Thomas
Answer: b
Explanation: Dalton proposed law regarding the partial pressures, that the total pressure that is exerted by non-reactive gases is the sum of the individual gas’s partial pressures. Ptot = ∑Pi , i = 1, 2, 3, 4, … n.
8. The partial pressure of a gas X is given by two bar, where is the total pressure of the gaseous mixture in a cylinder is 10 bar. What is the mole fraction of the gas X in that mixture?
a) 0.5
b) 2
c) 0.2
d) 5
Answer: c
Explanation: The partial pressure of a gas equals the mole fraction of the gas in the gaseous mixture x the total pressure that is exerted by the gaseous mixture. So here 2 bar = molar fraction x 10 bar, we get that the mole fraction is 2/10 = 0.2.
9. In a balloon of total pressure 6 atm there is a gaseous composition of 44 grams of carbon dioxide 16 grams of by oxygen and 7 grams of nitrogen, what is the ratio of nitrogen partial pressure do the total pressure in the balloon?
a) 0.25
b) 0.5
c) 0.75
d) 1
Answer: a
Explanation: The partial pressure of a gas is given by the mole fraction of the gas x the total pressure, so the ratio of the partial pressure to the total pressure is the mole fraction of nitrogen is 7/14 divided by 44/44 + 16/32 2 + 7/14 = 0.25.
10. Consider a gas of n moles at a pressure of P and a temperature of T in Celsius, what would be its volume?
a) nR(T + 273)/p
b) nRT/p
c) nR(T – 273)/p
d) R(T + 273)/p
Answer: a
Explanation: The ideal gas equation is given NY PV = not where p is pressure is the volume and is the number of moles are is universal gas constant and T is a temperature in Kelvin. So by arranging, we V = nR(T + 273)/p, as T is in Celcius, we need to add 273.

